In my previous article, I discussed a chemical found in both the great orange tip butterfly and the marble cone snail. I made the statement that the researchers were surprised to find that the chemical was identical in both species. A commenter asked a good question: When would the same chemical be different (across species or not)? I thought the best way to answer that question was with a new post.
When most of us think about chemicals, we think about simple molecules like water: H2O. The chemical formula of water tells us that there are two hydrogen atoms linked to one oxygen atom. In a glass of water, there are all sorts of molecules like this, and they are all identical. If we do something to change the chemical formula of the molecule, we come up with a completely different chemical. For example, if I were to add one more oxygen to the molecule, I would get H2O2, which is hydrogen peroxide. It is utterly different from water, so in molecules like these, even a change of one atom makes a world of difference.
However, the biological world isn’t quite the same. The molecules are incredibly complex, often composed of thousands of atoms. Consider, for example, proteins. These are large molecules made by linking smaller molecules, called amino acids, together. When amino acids link up together in a specific way, they tend to make a specific protein. An example would be the protein known as cytochrome c. It is a relatively simple protein found in almost all living organisms. It is simple because, as proteins go, it is rather small. In most living organisms, cytochrome c is composed of “only” about a hundred amino acids.1 That might sound like a lot, but there are proteins in living organisms that are composed of more than 25,000 amino acids!2 So as proteins go, cytochrome c is rather “simple.”
There are many ways to picture a protein, but one way is called a “ribbon diagram.” In this way of picturing a protein, you get a three-dimensional view of the overall backbone of the protein. Here is the ribbon diagram for cytochrome c:
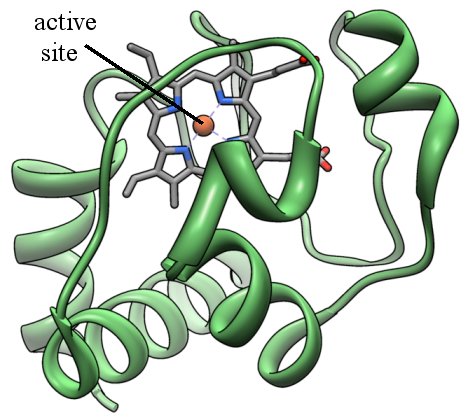
The green ribbons represent the structure of the backbone of the protein, and they are composed of many amino acids linked together. The gray bars represent the active site, which is where the protein does most of its work.
When a chemist looks at a protein, then, the most important part he or she sees is the active site. That’s what the protein uses to get its job done. For example, cytochrome c’s job is to pick up electrons that are produced when the cell is converting food molecules into energy. It then transfers those electrons to another chemical. Guess where it picks up the electron? At the active site, of course!
Now remember, cytochrome c is present in almost all living organisms. However, if I start comparing the cytochrome c in different species, I find some differences. For example, the cytochrome c in human beings is composed of 104 amino acids, but in plants, it is composed of only 100 amino acids.3 Clearly, then, the cytochrome c in humans has a different chemical formula from the cytochrome c in plants.
In addition, even when two organisms have cytochrome c molecules that are composed of the same number of amino acids, that doesn’t mean they are identical. Often, if I compare the specific arrangement of amino acids in the cytochrome c of different species, I will find that there are inconsistencies here and there. I might find that where one cytochrome c has the amino acid valine, for example, the other will have the amino acid isoleucine. Despite these differences, however, all these cytochrome c’s are considered the same chemical, because they do the same job at the same active site.
Why does the same chemical in different organisms have different chemical formulas? Because each organism has its own biochemical needs. As a result, an organism’s cytochrome c can be “tweaked” to make it work best within that organism’s biochemical framework. Generally, the structure of the active site is very similar from organism to organism, but the structure of the backbone is sometimes quite different. In the end, then, two organisms can have the same protein, but that doesn’t necessarily mean the two proteins are identical. They can be different from each other, and that difference usually reflects a difference in the overall biochemistry of the two organisms.
The chemical found in both the great orange tip butterfly and the marble cone snail is a “miniature protein,” called a peptide. It is not long enough to be considered a protein, as it has only eleven amino acids.4 Nevertheless, it has properties similar to that of a protein. As a result, one might expect that the number of amino acids or the sequence of amino acids would be slightly different between the butterfly and the cone snail, as is the case with many proteins. However, they are identical.
REFERENCES
1. N. V. Bhagavan, Medical Biochemistry, Fourth Edition, Harcourt Academic Press 2002, p. 255.
Return to Text
2. David L. Nelson and Michael M. Cox, Lehninger Principles of Biochemistry, Fourth Edition, W.H. Freeman and Company 2005, p. 87.
Return to Text
3. Concise Encyclopedia Biology, Thomas Scott, ed., Water de Gruyter and Company 1996, p. 351.
Return to Text
4. Hansson K, Ma X, Eliasson L, Czerwiec E, Furie B, Furie BC, Rorsman P, and Stenflo J., “The first gamma-carboxyglutamic acid-containing contryphan. A selective L-type calcium ion channel blocker isolated from the venom of Conus marmoreus,” J Biol Chem., 279:32453-32463, 2004
Return to Text
