In early June, an extraordinary paper was published in the journal Nature Communications. The paper is free to read, so I encourage you to take a look at it. The authors of the paper begin by offering a summary of the various discoveries of soft tissue in dinosaur bones. They then make this important point:
Models proposed to account for such preservation indicate that it should be the exception rather than the rule. In particular, it has long been accepted that protein molecules decay in relatively short periods of time and cannot be preserved for longer than 4 million years. Therefore, even in cases where organic material is preserved, it is generally accepted that only parts of original proteins are preserved and that the full tertiary or quaternary structure has been lost.
If you aren’t familiar with the terms, “tertiary” and “quaternary” structure refer to details that determine the three-dimensional shape of a protein, which is very important for its chemical function. Essentially, the authors of the paper are saying that the individual chemicals (called amino acids) that make up the protein might still be around after 4 million years or so, but the protein will be highly degraded.
So, the authors say that according to the prevailing wisdom right now, soft tissue preservation in dinosaur bones (which are supposed to be much, much older than 4 million years) should be very rare. They decided to test this idea, and not surprisingly, they found that it was wrong. How did they test it? They looked at eight dinosaur fossils found in Cretaceous rock, which is supposed to be between 145 million and 65 million years old. The authors suggest that the fossils are 75 million years old. The important point, however, is that these bones were definitely not well-preserved. As one of the authors said in a news report:
They’re very scrappy, individual broken bones. I can’t even tell you what dinosaur they come from.
What they found in these “scrappy” bones is surprising, at least if you think they are 75 million years old.
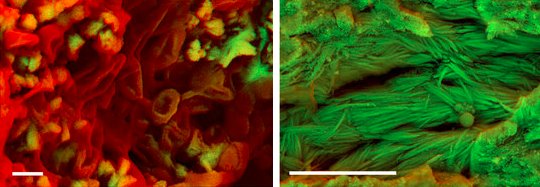
Two of the fossils yielded carbon-rich structures that resemble red blood cells (shown on the left in the image at the top of this post). All soft tissue is rich in carbon, which is mostly lost during fossilization. Given the fact that the structures are rich in carbon and have the shape of a red blood cell, the authors interpret them as unfossilized (soft) tissue. In addition, four fossils contained what appeared to be fibers of collagen, which is a protein (shown on the right in the image at the top of this post). Importantly, this is exactly what we would expect to see if the collagen’s tertiary and quaternary structures were still present. In other words, it doesn’t look like the protein has been degraded significantly.
Now, of course, the authors are very cautious in their interpretation, as they should be. Nevertheless, based on what they have reported, it really does seem like they have found soft red blood cells and intact collagen fibers in almost every one of the fossils they analyzed. What does this mean? Here is what the authors say:
Incredibly, none of the samples showed external indicators of exceptional preservation and this strongly suggests that the preservation of soft tissues and even proteins is a more common phenomenon than previously accepted.
In other words, the authors conclude that if they can find soft tissue in their “scrappy” fossils, there ought to be a lot of soft tissue in dinosaur fossils. Currently, there is no explanation as to how that can be, if such fossils are tens of millions of years old. Even Schweitzer’s attempt to claim that iron can preserve soft tissue requires an extraordinary set of conditions that would not occur very often. Indeed, the authors list that explanation as one which indicates that soft tissue preservation “should be the exception rather than the rule.”
If the authors are right, perhaps the best explanation is that such fossils are not tens of millions of years old.

Dr. Wile, have you read the latest CRSQ issue on their iDino C-14 dating of dinosaur bones? I haven’t gotten a copy yet, but Brian Thomas summarizes the results here: http://www.icr.org/article/8829
What I found most interesting–and I have heard this from other sources–is that they had to use third parties in order to get the tests done, because the labs won’t take their samples if they’re from a known creationist group.
Pretty spectacular. Combined with the Nature Communications article you cite here, the 65-75 million year old dates proposed for the fossils become harder and harder to justify.
I haven’t read the article yet, SJ, but I wrote about a talk that was given at a conference on that subject. You are correct that these labs, which are supposed to be engaging in science, try to censor these data rather than investigate them. Here is an example of what one lab wrote after they learned they were carbon-dating dinosaur bones:
This is the sad state of science today when it comes to origins. A lab that refuses to do scientific tests on certain samples is saying that those who want those tests performed have an “anti-scientific agenda.”
A very interesting article Dr Wile. It is especially interesting to note that the samples were very poorly preserved.
Dr Schweitzer reported that she saw the iron particles that she felt were responsible for preservation only on the transmission EM scans. I did not see any note of iron particles seen on the TEM scans done by this group.
Did you see any mention of this group seeing iron particles within the tissues when you read the paper?
They specifically mention that they could not find iron in the soft tissue, Bill. Now, they also say that the detection limit for their technique is pretty high. They used the same technique to detect iron in modern emu blood but didn’t detect it there, either. So they suggest that if there is iron in the soft tissue, it is on the order of 0.3% by weight. Now remember, Schweitzer used blood concentrate in her experiments, so one would expect the iron content was much higher in her experiment. Thus, one would have expected them to find some iron in the soft tissue, if it played a role in preservation.
The bigger piece of evidence against Schweitzer’s iron hypothesis is that, just like the amazing Triceratops soft tissue, the images of the soft tissue showed no iron particles. Remember, she saw iron particles embedded in the soft tissue she examined, which is what led her to the hypothesis to begin with. These images show no such particles. That seems to indicate that in these samples, iron played no serious role in preservation.
Thanks for the reply Dr Wile.
When I first read about Dr Schweitzer’s experiment that showed iron could preserve tissue I was concerned because she had used EDTA treated blood in the experiment. If you refer to the not so iron clad blog that you referenced in this blog you will find our conversation about EDTA and blood preservation near the end of the comments section. I wanted to let you know that I did run the experiment that I had suggested to see whether whole blood would preserve as well as blood treated with EDTA. The experiment lasted longer than I anticipated and the blog closed before I could post my final results.
The experiment was very simple. I obtained blood from my daughters dog and put part in a plain top blood collection tube and the remainder in an EDTA blood tube. It took longer than I thought for the untreated blood to degrade completely, but after about three weeks at room temperature in capped vials the untreated blood had almost no intact red blood cells in the smears I made to evaluate the samples. At that same time the EDTA blood vial was still producing smears that had reasonably good cellularity.
I realize my experiment was very informal and I have not repeated the experiment, but I suspect the results would be similar in additional experiments. For that reason I am still concerned that EDTA could have had some influence on Schweitzer’s experiment if some of the EDTA had been absorbed by the RBC’s in the treated ostrich and chicken blood.
Changing the subject, SJ referenced the CRS Quarterly issue on the iDino project. It is the spring 2015 volume 51 number 4 issue. There is an article titled Dinosaur Peptide Preservation and Degradation by DeMassa and Boudreaux. There is a significant amount of chemistry in the article and I would be very interested in reading your comments about the article and the chemical conclusions they have drawn about soft tissue preservation. Perhaps the article could even be the subject of a future blog article. Thanks again.
I remember that discussion, Bill. It’s great that you did the experiment, but I am not sure exactly what it shows. EDTA prevents blood clotting. Correct me if I am wrong, but wouldn’t clotted blood produce a smear with no cellularity? If that’s the case, then your experiment only showed that EDTA prevents clotting. In Schweitzer’s experiment, she was not looking at blood cells. She was looking at blood vessels, which don’t have a clotting mechanism. Thus, as I said in our previous discussion, I am not sure how EDTA could play a role in Schweitzer’s experiment.
I will definitely read the article you mention. If I don’t blog on it, I will at least let you know what I thought of it in this comment thread.
Thank you once again for your reply Dr Wile. Let me preface this discussion by saying that I highly respect the integrity of Dr Schweitzer as a researcher and a person. I am simply questioning the possible presence EDTA in the experiment because it is known to prolong the shelf life of stored blood. Because blood is a form of connective tissue, I personally consider that a form of tissue preservation.
My experiment was partially in response to your statement that “a preservative halts the decomposition process for some amount of time. EDTA doesn’t do that. If you add EDTA to blood, it will not coagulate, but it will continue to decompose just as fast as blood that has no EDTA in it.” What I intended to show with the experiment was that cellular lysis of the RBC’s was significantly more pronounced in the whole blood than in the tube with the EDTA. To me that is decomposition of blood.
You asked if clotted blood would produce a slide with no cellularity. The answer to that question is not initially. Initial samples of the clotted blood had significant cellularity for about two weeks. There was a gradual progressive decomposition of the untreated whole blood sample over about three weeks. During that time the EDTA treated blood maintained comparatively excellent cellular integrity.
You also mentioned that you were not sure how EDTA could play a role in Schweitzer’s experiment. Again, I am not sure that it did. My understanding is that blood clotting produces a destructive cascade. How the endothelial and other tissues of the blood vessels themselves are affected by that cascade would be a better question for someone with a forensic pathology degree. The question could be answered in the Schweitzer experiment by substituting whole blood for the EDTA treated blood and seeing if the experiment produced the same results. If it did not, I would be concerned that EDTA had influenced the initial experimental results.