The bones that make up the skeletons of animals and people are a marvel of engineering. As one materials scientist put it:1
…bone properties are a list of apparent contradictions, strong but not brittle, rigid but flexible, light-weight but solid enough to support tissues, mechanically strong but porous, stable but capable of remodeling, etc.
More than three years ago, I posted an article about research that helps to explain why bones are so strong. The calcium mineral that makes up a significant fraction of the bone, hydroxyapatite, is arranged in crystals that are only about three billionths of a meter long. If the crystals were much longer than that, the strength of the resulting bone tissue would be significantly lower. What restricts the size of the crystals? According to the previous research, the tiny crystals are surrounded by molecules of citrate. It was thought that the citrate latches onto the outside of the crystal, stopping it from growing.
Some very interesting new research from the University of Cambridge and the University College London indicates that this is, indeed, what happens. However, it also indicates that citrate does much more than simply restrict the size of the crystals. It also helps to produce a cushion that allows bones to flex rather than break when they are under stress.
Using nuclear magnetic resonance spectroscopy (the same basic technique used in an MRI) and X-ray diffraction, the authors show that the citrate which surrounds the crystals of hydroxyapatite in bone mix with water to form a gel that separates the crystals. This gel allows the crystals to slide against each other when the bone is stressed. In other words, the citrate/water mixture acts as a “shock absorber” that keeps bones from being too brittle. So not only does the citrate help to keep bones strong by reducing the size of the hydroxyapatite crystals, it also helps to keep them flexible by trapping water to form a shock-absorbing gel between the crystals!2
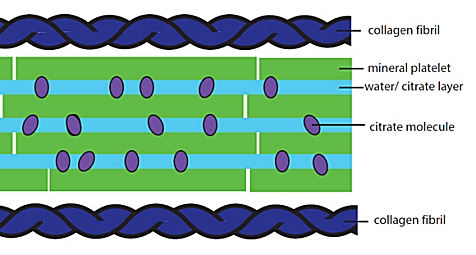
The authors’ new model for the microscopic structure of bone is illustrated in the drawing above. The citrate molecules essentially “hold on” to the water, keeping it from leaking out as it cushions the mineral platelets and allows them to slide against one another. The authors suggest that this model is important because it might explain the changes that occur to bones when people have metabolic diseases like diabetes. If the disease affects the way citrate is incorporated into the bones, it will change the tissue at a microscopic level.
What really impresses me is the dual role citrate seems to play in bone tissue. Not only does it contribute to the strength of a bone, but it also contributes to the bone’s flexibility. This is particularly amazing, because just a few years ago, no one had any serious ideas about why citrate is found in bone tissue in the first place. As the authors state:
Another factor that needs to be taken into account is the presence of citrate in bone. Its presence in bone is an accepted fact, but until 2010, there was no attempt to rationalize its presence within a structural model of bone mineral.
So in a matter of just four years, citrate went from a mysterious component of bone tissue to a molecule that plays at least two important roles in the tissue!
That’s what happens when you study incredibly well-designed systems. You keep uncovering new facets that demonstrate the ingenuity of their Creator!
REFERENCES
1. María Inés Sánchez Neira, “An efficient approach to the synthesis of a calcium phosphate bone-cement and its reinforcement by hydroxyapatite crystals of various particle morphologies,” PhD Thesis, Universidad de Santiago de Compostela, 2008 (Available online)
Return to Text
2. Erika Davies, Karin H. Müller, Wai Ching Wong, Chris J. Pickard, David G. Reid, Jeremy N. Skepper, and Melinda J. Duera, “Citrate bridges between mineral platelets in bone,” Proceedings of the National Academy of Sciences of the United States of America, 2014, doi:10.1073/pnas.1315080111
Return to Text

Several years ago I started taking calcium citrate because I was told that it is the most absorable form of calcium. Is that true and is that citrate the same substance you are speaking of?
Thanks for your question, Pam. The citrate in calcium citrate is the same as what is discussed in the article. When dieticians talk about how “absorbable” a supplement is, they are referring to how well it is absorbed through the small intestine and into the bloodstream. In order for the small intestine to absorb the calcium in any supplement, the supplement must be broken down into its constituent parts, which chemists call “ions.” Most calcium supplements (like calcium carbonate, calcium acetate, tricalcium phosphate, etc.) require acid in order to break them down, which is why it is recommended that you take them with food. The food stimulates the stomach to make lots of acid, and that helps break down the supplement.
Calcium acetate doesn’t require acid to break down. Thus, it can be taken with or without food, and it is especially useful for those who take acid blockers or those whose stomach has trouble producing acid. When taken with a meal by people with normal stomachs, however, calcium citrate is no more absorbable than any other calcium supplement. When taken with no food or just a little food, it is more absorbable than the other supplements.
Your body makes citrate, so I doubt that the citrate in the supplement is used in bone-building. However, I don’t know of any studies about that, so there could be some mechanism of which I am not aware. Certainly with the new role that citrate has been found to play in bone tissue, it couldn’t hurt to take citrate with your calcium!
Dr. Wile,
I’ve been following your blog for a few months now and I really enjoy it. Also I’ve read your General Science and am reading your Physical Science right now. They’re really good. Thank you for the work you do and have done.
Today I ran into this post about chicken broodiness that I thought might interest you: http://www.backyardchickens.com/t/869117/new-byc-sponsored-study-increase-chicken-broodiness-by-up-to-48-with-aluminum-foil
I was wondering what you thought about it. The hypothesis is kind of strange in my opinion.
Thank you and God bless!
Abigail
Hi Abigail. Thanks for your kind words about my courses!
It’s always fun to read such reports and try to see how the author tells you it is a joke. I think the reference to the “Federal Animal Testing” association (FAT) is one way. Also, the reference to a 401-page research paper is another. It looks like the author had a lot of fun, especially with some of the commenters.
Oh, they edited it later to say it was April fools! I didn’t know. I was fooled.:) Well, thank you anyway.
It would be easy to be fooled. The article was written very well!
The way bones (and the human body, for that matter) are designed continues to amaze me. Do you think there will ever be any structures engineered that are similar to bone, i.e. utilize similar design characteristics like the one mentioned in your article? Do such man-made materials already exist?
It depends on what you mean, Nathaniel. When medical scientists want to produce artificial bone for use in patients, they typically want to make a scaffold on which actual bone tissue will eventually grow. Ideally, that scaffold will dissolve as the bone tissue forms. The most promising process in that arena seems to come from 3-D printing. If you are talking about materials that are used completely replace bone, the most promising process is inspired by sea shells. If you are talking about building materials used in other applications that have properties similar to bone, the most promising process comes from mimicking bone’s structure. None of these has all the properties of bone, however.