One of the problems that science textbook authors face is the fact that science is constantly changing. As we learn more about the Creator’s handiwork, we find that the science we have taught as fact is actually incorrect. Sometimes, this is because the experiments upon which those facts are based were in error. Sometimes, it’s because our interpretations of those experiments were in error. Sometimes, it’s a result of making conclusions that go beyond what the experiments actually tell us. The practical upshot of all this is that some of the things you are reading in your science textbooks are wrong.
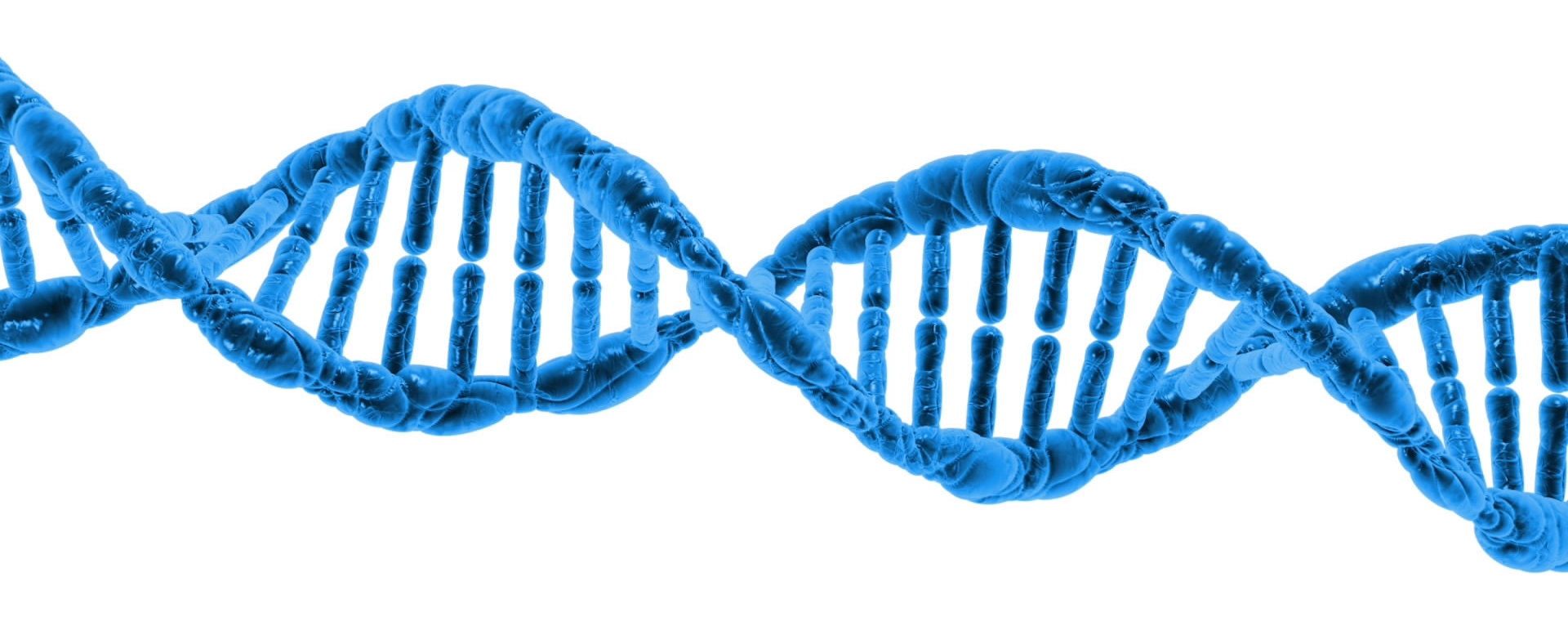
I recently found out that something I (and most other authors) have taught about DNA is probably wrong. Most people know that DNA is a double helix. As shown in the illustration above, those two helixes wind around each other, with the information-bearing units (called nucleotide bases) inside. In order for cells to use the information in DNA, those helixes have to be separated so that the sequence of the nucleotide bases can be read. That means the helixes need to be held together when DNA is not being used, and then they must be separated when it is time for the cell to read the DNA.
How does that happen? Well, according to most textbooks (including mine), it is because the nucleotide bases form hydrogen bonds with one another. Hydrogen bonds are weaker than true chemical bonds, but they can hold things together. As I say it in my textbook, Exploring Creation with Biology, 2nd Edition:
…the attraction between the atoms in hydrogen bonding is about 15% as strong as the attraction between two atoms that have a true chemical bond linking them. Thus, the hydrogen bonds in DNA are strong enough to keep the two chains together in a double helix, but they are significantly weaker than a true chemical bond. Since they are weaker than a true chemical bond, it is rather easy for the two helixes in DNA to unravel.
This sounds great, but a recent study indicates that it’s probably not true. If nothing else, it doesn’t tell the entire story.
The researchers showed that you can unravel the two helixes in DNA without forcing the hydrogen bonds to break. Instead, all you have to do is change the environment in which the DNA exists. If DNA is in an environment that is mostly water, the two helixes hold together nicely. This is because DNA is hydrophobic, which means it is repelled by water. The DNA stays together in order to avoid the water molecules as much as possible. However, if you add a chemical that makes the DNA’s surroundings more hydrophobic, the DNA will relax, because it doesn’t need to avoid as many molecules. If you make the environment hydrophobic enough, it will relax to the point that the helixes unravel.
Based on their results and results from other researchers, the authors suggest that there are enzymes in the cell’s nucleus that do exactly that. When the DNA is not being read, it is surrounded by a lot of water molecules, so the helixes stay tightly wound around one another to avoid the water molecules. When the cell needs to read a section of DNA, enzymes are brought near that part of the DNA. Those enzymes are partially hydrophobic, and the DNA relaxes. This allows the helixes to unravel so that the information can be read.
This research really surprised me, since I have used the textbook explanation over and over again throughout my years as a science educator. Because of this, I contacted a molecular biologist who is a part of my extended family. I don’t like to bother him too often, because he is retired, but he is one of the most accomplished, brilliant, and humble scientists I have ever met. I asked him if he would take a break from fishing and hunting to let me know what he thought of this “surprising” research.
He basically said that he didn’t find it terribly surprising. He said that years ago, his lab saw that adding a chemical called formamide significantly lowers the temperature at which you need to heat DNA to unravel the helixes. This was good for them, because they wanted to heat the DNA as little as possible. They assumed it was because the formamide was making the DNA’s surroundings more hydrophobic and therefore allowing the DNA to relax, reducing the energy needed to pull the helixes apart. They just never investigated the process to see if they were right.
Now this doesn’t mean the hydrogen bonds between the nucleotide bases in DNA are not important. They allow the bases on each helix to pair up properly, and they may aid in holding the two helixes together. However, the textbook explanation that they are the main reason DNA holds its double-helix shape when it is not being read is almost certainly wrong.
Science is a wonderful way to gain knowledge about God’s creation, but by its very nature, it is tentative. You must always be willing to reevaluate what you have been taught about science, because much of what you have been taught will eventually be shown to be wrong.

Do you think this will have any impact on the research into ancient DNA in fossils?
I would think so. Correct me if I am wrong, but I would think that most fossils start by being covered in wet sediment, so for at least some time, they are in an aqueous environment. That would mean the DNA is held together better, which would reduce the amount of decay. Of course, there are probably a bajillion other factors, but for this specific issue, I would think that fossils formed in a more aqueous environment might hold less degraded DNA.
Fossil formation requires organic remains to be buried rapidly and deeply enough to keep them from being scavenged or degraded. If you think about it, it’s amazing that any fossils exist at all, because the earth’s ecosystem is so good at removing dead things from its surface.
That’s why I think a global flood is the most logical explanation for the billions of fossils that exist: 1) the dominant sedimentologic process on the earth nowadays is erosion, not deposition, so mass fossilization required a fundamentally different ratio of deposition to erosion than what exists today, and 2) fossils are found worldwide, so processes causing mass fossilization had a global extent. Both those factors are much more consistent with a catastrophic global inundation than with a projection into the past of the sedimentary processes we see today, even allowing for local catastrophes.
Nice post, Dr. Jay! And the last paragraph is so very true that I have no words; that is basically what separates an truly seeker of the truth from a narrow viewed agenda driven “scientist”.
God Enlighten you!
Let me just add a disclaimer here: Before anyone ask or say(in this case, write), I’m pretty aware that we, as humans being(sadly) are not bias-free. The point I was saying is that some people act 100% based on him/her bias; while others act 50%, etc.
And I have full knowledge pretty much all studies made by consecrated scientists or one that just started the university have some personal self interest on it(for selfless or selfish reasons).
Therefore when you see at the end of a paper the section “conflict of interest”, don’t take it to the latter.
God Enlighten you all!
Thanks for sharing this. I’m wondering what you do when things in a book you’ve already published are proven untrue…other than of course sharing about them on your blog. I was thinking, in this internet age, it would be cool to have a corrections page showing what parts of your book needs to be updated with more recent science, so that people who have it already can just add that part (as a homeschooler, I print out stuff and just tape it over, or just write in the book if it’s a short correction).
Most texts have a website that goes with the book. Authors generally post things like that there. Some publishers are more diligent than others about letting people know how the science changes.
This has been known for decades. In fact, it has been proposed that methylation can silence genes by increasing the hydrophobicity of the methylated strands of DNA. It’s not that science has recently discovered something new about hydrophobicity and DNA…. its that YOU have recently discovered something about DNA that others have already known for a long time.
(I’ll note that the article you cite is about DNA stacking, which happens after the strands are bound to each other in a helix. The article doesn’t really address the bond between the two individual strands, although you imply it does.)
That doesn’t make this essay uninstructive. To the contrary, it drives home the point that people who write and teach about science should make sure that they stay abreast of recent developments.
If this is new, why does this website posted in 2003 describe hydrophobicity as a major force in keeping the two DNA strands together?
https://cnx.org/contents/k6E8Y6SA@8/Primary-DNA-Molecular-Structure
I’ll tell you why – this has been known for a very long time. Just because it’s new to you doesn’t mean it’s new to science.
I am not sure why you think I am saying the idea is new. Note what I specifically say:
All I am saying is that is IS news to me, and the way I learned about it is through a recent study. In fact, I specifically say:
This, of course, is because the authors specifically address previous research that supports the idea. That implies it is not new. However, as I say in the article, it is new to me, and I learned about it from a recent study.