Yesterday, President Trump held a press conference to announce a new possible treatment: convalescent plasma. Based on an analysis of several different studies, it seems to be the best candidate yet (in my non-medical-doctor opinion). I say this because of the kinds of studies that have been done. First, there have been three randomized clinical trials. This means patients were assigned to either get the treatment or not get the treatment based on random chance. The group that got the treatment was compared to the group that didn’t (called the control group). In the three studies, the death rate in the treatment group was half that of the control group. There were also five matched-control studies, where the treated patients were compared to a control group specifically selected to closely match them. The results of those studies were similar to those of the randomized clinical trials. There were also four case-series studies, where patients were given the treatment and their progress was tracked. While that kind of study has practical uses for physicians, its ability to determine the effectiveness of a treatment is extremely limited. However, the case-series studies seem to support the other two kinds of studies. All of the studies were done on patients with severe or life-threatening cases of COVID-19.
Taken all together, then, the treatment looks very promising. However, I do have to say that each study was very small, so even when all the patients were analyzed, the total number was only 804. Phase three clinical trials that determine whether or not a drug should be widely used typically involve a few thousand patients. Thus, this is still a limited data set. Also, many of the studies (as well as the analysis linked above) are not peer-reviewed. As a result, there could be major flaws that have not been noticed. A recent analysis (once again not peer reviewed) of more than 35,000 patients seems to support the small studies, but since it has no control group, it cannot be used to draw any real conclusions. Nevertheless, the FDA has approved emergency use of the treatment, and it is asking those who have recovered from the disease to help in determining whether or not it is truly effective.
How can someone who has recovered from COVID-19 help determine the effectiveness of the treatment? To understand that, you need to learn a bit about the wonderful mixture that is running through your circulatory system.
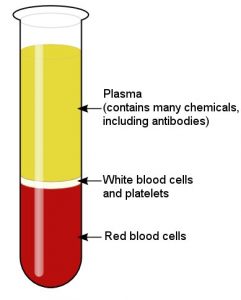
As shown in the image above, blood is a mixture of three easily-separated components. The first component is made of red blood cells, which carry oxygen from the lungs to the tissues. These cells also give blood its red color. Second, there is a component made up of white blood cells (which help to fight infections) and blood platelets (which help in blood clotting). The largest component is the blood plasma. Making up about 55% of the blood, it is a water-based solution that carries all sorts of chemicals. It holds energy-producing chemicals (like glucose), vitamins, and a wide range of proteins. This wide range of proteins includes antibodies, which are produced by your immune system to attack invaders (like bacteria and viruses).
If you have recovered from COVID-19, your body must have made antibodies to attack the virus. Since you recovered, the antibodies were clearly effective, so the idea is that if you donate blood, the plasma can be separated from it and given to someone who is currently fighting the disease. The antibodies in your plasma might be able to do what they did inside you – help the patient’s body fight the virus. This is called convalescent plasma treatment and has been used successfully for more than 100 years, but its effectiveness varies considerably, depending on the illness. Thus, while it makes sense that the treatment should work, we don’t know how well it works for COVID-19, even though the initial studies show promise. We also know that it is a safe treatment, since it has been used for so long in so many situations.
If you have recovered from COVID-19, you might consider helping to determine whether or not this treatment is truly effective. All you have to do is donate some of your blood plasma. The donation center will have to confirm that you still have antibodies, because it’s possible that you recovered from COVID-19 but no longer have them. Antibodies are complex chemicals that tend to decay. Your body might not make them once it no longer needs them, so there are people who have recovered from COVID-19 that do not have antibodies in their plasma. That doesn’t mean they are at risk for getting COVID-19 again, however. Antibodies are made by specific white blood cells, and those cells survive long after the antibodies. If the body sees the virus again, they will make new antibodies right away. As a result, current research indicates that people who have recovered from the disease will have long-lasting immunity to it.

Costa Rica, Brazil and Argentina are going to use plasma from horses. Scientists says production is more efficient, because you can get more plasma from a horse, and it seems to give better results than convalescent plasma and is quite similar to the treatment of snakebite poisoning. The solution successfully passed the tests run by George Mason University in the United States.
https://www.scientificamerican.com/article/costa-rica-readies-horse-antibodies-for-trials-as-an-inexpensive-covid-19-therapy/
A breath is light in the darkness of one more politicized disease. Thank you!
What about those that have recovered from COVID-19 and later became ill from the same virus? Could that be caused by a slight mutation or an underlying condition that may have been triggered by the initial infection?
Speaking of trials, when it comes down to giving people medication, what’s the purpose of offering placebos? From my understanding it’s a way to test if something actually worked.
By the way, good article.
Thanks, Ken. I have not read any studies regarding people who get it twice. I am skeptical that it really happens, but I just haven’t seen the relevant data.
A placebo makes subjects think they are getting medicine. The power of suggestion is strong, so you have to take that into account. So…you give some people a placebo and others the medicine. That way, the only difference between the groups is getting the medicine. Then, when you compare the groups, you can accurately determine the effect of the medicine.
Interesting, I’d never heard of this treatment before.
If someone received convalescent plasma treatment, do they need to receive plasma from someone with the same blood type, or not?
Also, would this type of treatment work for someone with COVID 19 if one were to receive pure blood from someone with the same blood type who recovered from COVID 19?
They would need to have the proper type, because the antibodies that target blood type are in the plasma. I do not know of any studies that have seen if whole blood has the same effect, but I would think that it should, since it has the plasma as well.
Seems to me that IF whole blood had the same effect, it would save time and effort removing the plasma.
Unless there are other factors I’m overlooking. Perhaps plasma has a longer shelf life than whole blood.
Shelf life is one reason, but also volume. Your body can’t accommodate too much volume, so by giving just plasma, you can give more antibodies per unit volume.