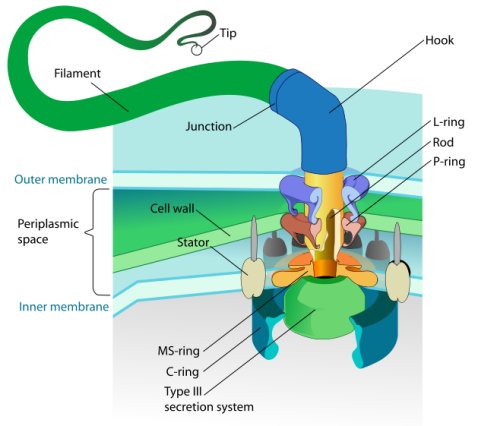
The bacterial flagellum is a symbol of the Intelligent Design movement, and rightly so. After all, bacteria are commonly recognized as the “simplest” organisms on the planet. Nevertheless, their amazingly well-designed locomotive system has continued to amaze the scientists that study it. In 1996, Dr. Michael Behe highlighted the intricate design of the bacterial flagellum in his book, Darwin’s Black Box. While some have tried to explain it in terms of Neo-Darwian evolution, they have not come close to succeeding.
Not only is the bacterial flagellum amazingly well-designed, it is far more versatile than anyone imagined.
Some bacteria (like Escherichia coli) have multiple flagella, which makes it very easy for an individual to navigate in water. All the bacterium has to do is adjust which flagella are spinning and how they are spinning, and the single-celled creature can do acrobatics in the water. However, the vast majority of bacteria have only one flagellum. It was thought for a long time that because of this, it is difficult for them to make sharp turns in the water.
Two years ago, this thinking changed abruptly when a group of physicists from the University of Pittsburgh showed that the bacterium Vibrio alginolyticus, which has only one flagellum, can make sharp turns with ease. They showed that in order to execute such a turn, the bacterium backs up, lurches forwards, and swings its flagellum to one side.1 The entire maneuver takes less than a tenth of a second and results in a 90-degree turn. So not only is the bacterial flagellum an exquisite “outboard motor” that propels the bacterium through the water, it is also a rudder that allows the bacterium to make sharp turns at will!
Continue reading “The Bacterial Flagellum: More Sophisticated Than We Thought!”