In 2005, Dr. Mary Schweitzer stunned the paleontology community by finding soft tissue in a Tyrannosaurus rex fossil that is supposed to be more than 65 million years old. Because it is very difficult to understand how tissue could remain soft for more than 65 million years, many scientists tried to contest her findings. Over the years, however, more discoveries of soft tissue in fossils that are supposed to be multiple millions of years old have been made (see here, here, here, here, and here, for example). As a result, most scientists have come to accept the fact that there is soft tissue in fossils that are up to 550 million years old.
Now the focus on soft tissue in fossils is changing. Scientists are trying to find some chemical mechanism that would allow soft tissue to avoid decay and fossilization over such a long period of time. Dr. Schweitzer herself did experiments to suggest that iron might help to stave off decomposition and fossilization, but from a chemical standpoint, it simply doesn’t work (see here and here).
A reader recently asked me about another proposed explanation that I had somehow missed. The study was published late last year, and while it attempts to explain how soft tissue can avoid decomposition over millions of years, it doesn’t achieve its goal. Instead, it actually gives more evidence that the fossils in the study are very young. However, it does produce some interesting results that require further investigation.
To understand the study, you first have to learn two chemical terms: oxidation and reduction. In short, oxidation occurs when an element in a chemical reaction loses electrons. Reduction occurs when an element gains electrons. As you can see, these processes are “opposites” of one another. As a side note, batteries are based on chemical reactions that use oxidation and reduction. Well, some environments (especially ones that have some oxygen in them) can perform oxidation on the chemicals found in that environment. Not surprisingly, such environments are referred to as oxidative environments. An environment that tends to reduce the chemicals in the environment are called reducing environments. Now you are ready to understand the study.
The researchers looked at several fossils. Based on their external characteristics, they decided whether the fossils were exposed to an oxidative environment or a reducing environment. They then crushed up samples of the fossils and soaked them in an acidic solution (pH = 1). The acid removed the minerals in the fossil, but it wasn’t strong enough to react with any soft tissue that might be in the fossil. They found that while none of the fossils they thought came from reducing environments had any soft tissue in them, several of the fossils they thought were from oxidative environments did. In other words, this study seems to indicate that oxidative environments yield fossils with “soft” tissue, while reducing environments do not. This should be investigated further to see if it is a general trend.
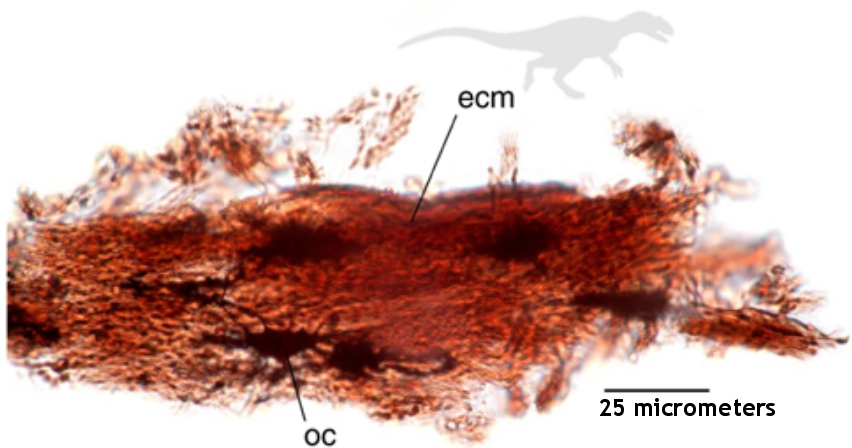
The reason I put “soft” in quotes is that while these tissues were not mineralized, they were not soft and flexible, either. For example, the authors found tissue in fossil scales as well as bones. They describe the tissue from the scales as “brittle and cracked” and the tissue from the bones as “also brittle.” Thus, the “soft” tissue they found isn’t like the amazingly elastic soft dinosaur tissue that Dr. Schweitzer found or that Dr. Anderson and Mr. Armitage found. Despite the tissue not being what one normally thinks of when it comes to soft tissue, it was clearly tissue that had not been fossilized, so it is a kind of soft tissue.
They examined their “soft” tissue with Raman spectroscopy, a well-known technique which allows you to learn about the nature of the molecules in a sample. They found lots of Advanced Glycoxidation Endproducts (AGEs) and Advanced Lipoxidation Endproducts (ALEs). Those are what you expect to find when proteins undergo oxidation in biological environments. That’s not really surprising. Put a tissue sample in an oxidative environment, let it sit, and it will form AGEs and ALEs at the same time it is decomposing. Indeed, the researchers confirm this by taking soft tissue from modern eggshells, bones, and scales and heating the tissue in an oxidative environment while continually moistening it. This produced some AGEs and ALEs, but not as many as what was seen in the “soft” tissue that came from the fossils.
The researchers used these results to suggest that this might be how soft tissue remains in fossils after millions of years. The proteins undergo oxidation to form AGEs and ALEs. Those chemicals retain the shape of the soft tissue, but they are significantly more resistant to decomposition than the proteins that they came from. In addition, AGEs and ALEs tend to repel water, so they might form a water-resistant “shield” around tissue that is deeper in the fossil, allowing it to resist decomposition even better.
AGEs and ALEs are more resistant to decomposition than proteins, but there is no indication that they would last for millions of years. There is certainly no evidence for that given in the paper. They are also water-repelling, but it’s not clear how they would shield other tissue from water. They would have to fully form on the outer parts of the fossil before water got to the inner parts of the fossil, and it’s not clear how that would happen. Nevertheless, I would be interested in any proposed mechanisms by which this could occur.
But none of that is nearly as important as something the authors of the paper don’t seem to notice. Their Raman spectroscopy of the fossils demonstrates that the proteins in the tissue are not fully oxidized. Yes, parts of the proteins have oxidized, but parts of them have not. In Figure 2, for example, the spectra clearly show that the Allosarus fossil that is supposed to be 150 million years old still has many of the original protein bonds intact. Sure, there are fewer intact protein bonds in the fossils than in the modern tissue that was heated, but still, there are plenty of intact protein bonds. If the tissue were millions of years old, I wouldn’t expect that!
Consider, for example, the way they got AGEs and ALEs to form in the modern tissue. They heated it. Heating speeds up the oxidation, but the highest temperature they used was 120 degrees Celsius, and the longest they heated the tissue for was 60 minutes. In that very short time, a lot of oxidation had already occurred! That’s what I would expect. Now, of course, the fossils weren’t exposed to such high temperatures, but they are supposed to have millions of years over which the oxidation could take place. Why aren’t the proteins fully oxidized?
Indeed, one of the samples they looked at was an eggshell fossil from Egypt. It is thought to be 3,000 years old, and it is fully oxidized. No original protein bonds are detected in the “soft” tissue pulled from that fossil. As a chemist, that’s exactly what I expect. Under typical conditions, I would think that the proteins in a fossil would fully oxidize in a few thousand years. Now, if conditions are particularly good, it might take twice as long, or perhaps ten times as long. However, to get the results that these researchers got, you have to believe that oxidation in the Allosarus fossil takes more than 50,000 times as long as it did in the Egyptian eggshells. As a chemist, I cannot find any way to justify that.
In the end, then, while this study is an attempt to explain how soft tissue can avoid decomposition and fossilization over millions of years, the results provide even more evidence that the fossil tissues which were examined are only thousands of years old. I do hope more studies like this one are done so we can have a better idea of how much oxidation has taken place in dinosaur fossils. I predict that this will further strengthen the case that the fossils are not millions of years old.

Dr. Wile,
Great article! Also, I would think measurements of amino acid racemization would be in order. The proteins in fossils have a high level of residual homochirality, when racemic heterochirality is the expectation after millions of years. Even with adjustments using the Arrhenius equation, the fossils look too young.
I hear it’s not a hard technique to measure racemization. I’m not a chemist, however, just a trouble maker.
Your article adds yet another clock to examine regarding fossil dating.
Good article, Dr. Wile, but I could not help but notice that Dr. Armitage was not properly referred to in you article or properly attributed as the senior author in the ACTA paper. Dr. Anderson was included as a professional courtesy. Is there an issue between you and Armitage?
Thanks, Tom. I mention Dr. Anderson first because I was linking to my review of Is Genesis History, which features Dr. Anderson. I have no problem with Mark Armitage. In fact, I have written glowingly about his work (see here, here, here, here, and here). Indeed, P.Z. Myers hated the praise I heaped on Armitage (see here and here).
I was longing for this theme to pop up again! I really enjoy it!
Godspeed, Dr. Jay!
Very interesting. I remember reading Schweitzers peer saying something to the effect of “All Hell Creek fossils smell”. I think that’s a good place to start. What is different about that area? Why are those fossils prime candidates for soft tissue?
Much thanks. Very good read and I posted it on FaceBook. I’m waiting to hear from biologists in the family on it. But, I recall reading an article years ago about soft tissues being discovered in the early 1960s. Any ideas on that? Niio!
The first I heard about soft tissue in “ancient” fossils was Schweitzer’s 2005 paper.