Author: Kevin Spear
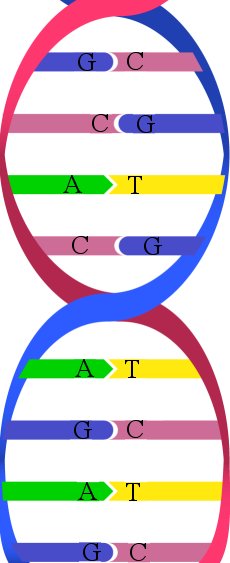
One of DNA’s elegant features is that the nucleotide bases are linked together with hydrogen bonds. Unlike their name implies, hydrogen bonds aren’t really chemical bonds at all. Instead, they are very strong attractions that exist between a hydrogen atom on one molecule and another atom (typically oxygen or nitrogen) on another molecule. Because hydrogen bonds are not true chemical bonds, they are not nearly as strong as chemical bonds. As a result, they can be “broken” with only a small amount of energy.
It turns out that this is the perfect design, because in order for DNA to code for proteins, the double helix must “open up” to expose the nucleotide bases. To do this, the link between the nucleotide bases must be broken. If the nucleotide bases were held together with chemical bonds, it would take a lot of energy to break the link, and that energy could easily damage the other bonds in DNA. Since the nucleotide bases are linked with hydrogen bonds, however, it takes only a small amount of energy to break the link. As a result, DNA can “open up” very easily, and the rest of the molecule is not harmed when that happens.
Watson and Crick determined all this, including exactly where the hydrogen bonds formed. Not surprisingly, the way in which the links form is called Watson-Crick pairing. Well, it turns out that there is another way the nucleotide bases can pair up, and a recent study shows that this is yet another amazing design feature of DNA.
Continue reading “DNA Is Even More Complex Than We Thought!”