(public domain image)
When I first heard about the idea that radioactive decay might vary from the smooth, constant-half-life behavior that is typically observed, I was more than a little skeptical. As a nuclear chemist, I am well aware of how much energy it takes to affect nuclear processes. Since those energies are not generally attainable except with the use of a particle accelerator, a magnetic containment system, or some other high-powered device, it seemed absurd to think that variable radioactive decay was anything other than the mad wish of those who didn’t like the conclusions of radiometric dating. However, over the years, the data have convinced me otherwise. I written a couple of posts about variable radioactive decay (see here and here), and it seems clear to me that it does happen, at least under some circumstances.
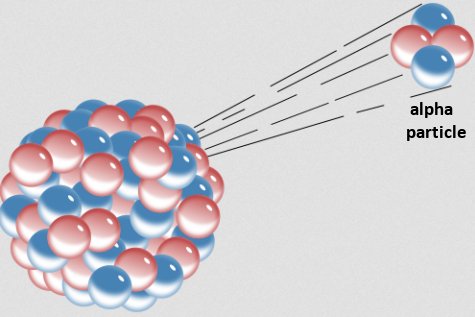
Recently, I came across another study on variable radioactive decay. It is actually a follow-up to a previous study,1, and it explores the alpha decay of uranium-232. As shown in the drawing above, alpha decay is one specific type of radioactive decay in which an unstable nucleus attempts to reach stability by spitting out two protons and two neutrons. Those four particles are bound together to form the nucleus of a helium atom, which for historical reasons is also called an alpha particle. It turns out that when uranium-232 does this, the resulting nucleus still isn’t stable, so a long series of further alpha decays occur, eventually producing lead-208, which is stable.
The authors of the study I am writing about weren’t interested in the subsequent decays. They looked specifically at the alpha decay of uranium-232. Under normal circumstances, this decay has a half-life of 69 years.* This means if I start with 200 uranium-232 atoms, after 69 years, only half of them (100) will remain. The other half will have decayed away. If I wait another 69 years, only half of those (50) will remain. In another 69 years, half of those (25) will remain. In the end, this is typically how radioactive decay works: the number of radioactive atoms ends up decreasing by half over every half-life.
The results of the study seem to indicate that a tabletop device involving a laser and gold can end up decreasing the half-life of uranium-232 by as much as a factor of 435,494,880,000,000!2