(Public domain image)
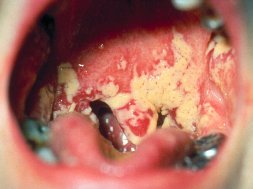
People want a strong, light, and porous material, which is almost a contradiction in terms, but nature does it…Bone is made from calcium phosphate and collagen, which are both extremely weak. But nature mixes them together at room temperature and without toxic chemical [sic] to create something that is very tough — this fascinates us.
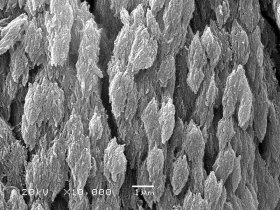
What makes bone so special? The short answer is that we don’t really know. However, we are learning. For quite some time now we have known that bone is a mixture of many things, principal among them a protein called collagen and a calcium compound called hydroxyapatite. The collagen gives bone its flexibility, while the hydroxyapatite gives bone its strength.
However, the hydroxyapatite in bone is stronger than hydroxyapatite made in the lab. Why? It has to do with the size of the crystals. When hydroxyapatite is made artificially, the individual crystals that form are very large. In bone, the crystals are very small, on the order of 3 billionths of a meter long. These nanocrystals have long been thought to be the reason that hydroxyapatite in bone is so strong. However, scientists haven’t been able to understand why the nanocrystals stay so small in bone.
Now Klaus Schmidt-Rohr and his colleagues might just have figured that part out!