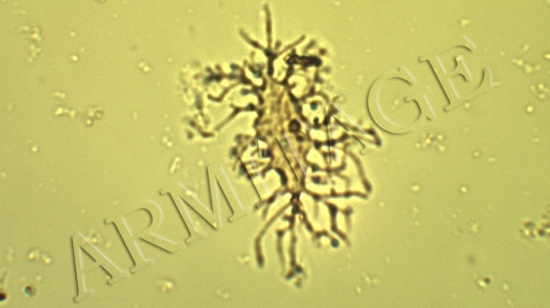
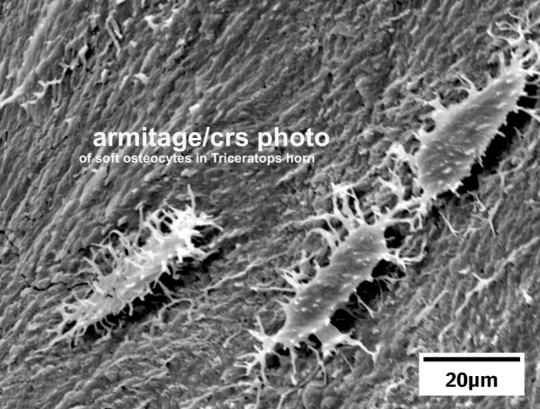
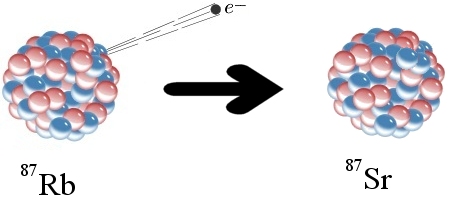
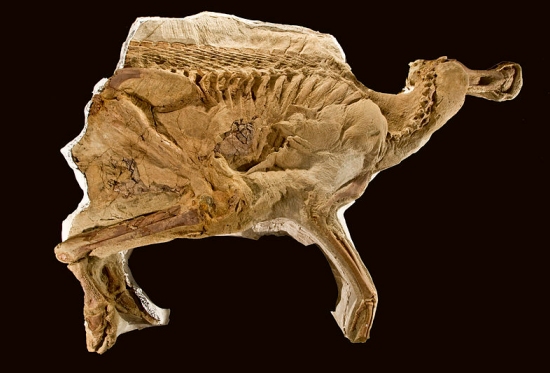
Six years ago, I wrote an article about Anderson University, where I am an adjunct professor. While the university clings strongly to the essentials of the Christian faith, it does not force its faculty to conform to one interpretation of Scripture. As a result, students are exposed to many different views that exist within Christendom.
In addition, rather than just trying to proselytize for their own view, the faculty are committed to making sure students understand the different ways Christians interpret the world through the lens of Scripture. This is best exemplified by an example. One of the science professors is an old-earth creationist, but he regularly invites me into his classes either to give a young-earth view of the science the students are learning or to engage in a friendly debate with him on the issue of the earth’s age. I especially like the latter, since students see that two people can engage in serious disagreements and still be good friends.
Just before Christmas, someone I respect and admire sent me an article that I wanted to share with my readers. It gives you another example of a Christian College (in this case, a seminary and Bible College) that gets it. To fully appreciate the article, however, you need to know the history behind it.