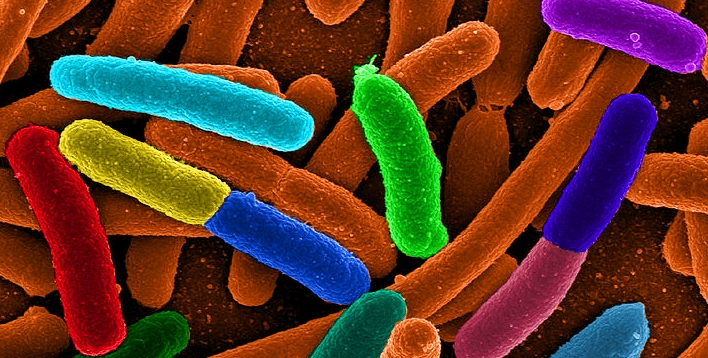
Escherichia coli (E. coli) are the workhorses of the bacterial world. They are found in every human being and most warm-blooded animals, but they are also found in laboratories all over the world. Because they are easy to care for, reproduce quickly, and have a genome that is reasonably well-understood, they are a popular subject of study among biologists. In addition, they end up producing a lot of chemicals that we need but are unable to produce ourselves. For example, insulin is a protein that all people need, but some people don’t make enough of it (or don’t respond well enough to it) to remain healthy. That leads to diabetes, and one treatment for diabetes is regular insulin injections.
While the insulin in pigs and cattle is close to what we find in people (and was used to treat diabetes for a long time), the best insulin for most diabetics is human insulin. Unfortunately, even with the best technology available, we aren’t good enough chemists to make insulin, but simple organisms like bacteria are. As a result, scientists have learned how to insert the human gene for insulin into a bacterium, which allows the bacterium to do the chemistry for us. As a result, much of the insulin used to treat diabetics today is human insulin produced by E. coli bacteria.
Unlike some species of bacteria, however, E. coli have to eat in order to get the energy and raw materials they need to do that chemistry. This ends up producing carbon dioxide as waste. To reduce the buildup of carbon dioxide in the atmosphere, then, it would be nice to produce chemicals like insulin from an organism that does not produce carbon dioxide in order to live. There are technical problems with that, however, so right now, diabetic insulin (and many other medically-related chemicals) adds to humanity’s “carbon footprint.” There are two ways to fix this: Either figure out how to use organisms that don’t have to eat (like organisms that make their own food through photosynthesis) or change E. coli so that it doesn’t have to eat.
In a recent study, scientists have been working on the second alternative and have experienced some success. As a byproduct, they have produced something that can be used to test creationism.
Continue reading “A Bacterium That “Eats” Carbon Dioxide…and a Creationist Prediction”